Clinical Trials refer to planned research studies conducted on human participants to explore biomedical or behavioural interventions. Clinical Trials in India are conducted in 4 phases. In this article, you will learn definition of clinical trials, various phases of clinical trials, history, importance, objectives, etc.
This article will provide key insights for GS Paper-3 Science and Technology of UPSC IAS Exam.
Table of Content
- What is a Clinical Trial?
- Legislation for Clinical Trials in India
- What are the phases of clinical trials?
- Process of Clinical Trials in India
- Consent-Ethics Committees
- Key Issues with Clinical Trials
- Way Forward
- Conclusion
- Frequently Asked Questions
What is a Clinical Trial?
- Clinical trials refer to planned research studies conducted on human participants to explore biomedical or behavioral interventions in order to address questions related to new treatments like novel vaccines, drugs, dietary choices, dietary supplements, and medical devices.
- It also addresses questions related to established interventions that require further investigation and comparison.
- These trials are essential for gathering data on dosage, safety, and effectiveness of the interventions under study.
- This concept has originated after the Nuremberg Trials during World War II and after which the Nuremberg Code 1947 was adopted.
- International Clinical Trials Day is held on May 20 each year to commemorate the day that James Lind began the first randomized clinical trial in 1747.
- The inclusion criteria identify the study population in a consistent, reliable, uniform and objective manner.
- The exclusion criteria include factors or characteristics that make the recruited population ineligible for the study.
- Difference between clinical research and clinical trials is that clinical research could be prospective or retrospective, but clinical trials may be initiated to find treatment, prevent, observe, and diagnose a disease or a medical condition.
- Clinical trial performance metrics provide information across systems to track execution, manage logistics, and detect risks across multiple sites and regions.
Types of clinical trials:
- Pilot studies and feasibility studies.
- Prevention trials.
- Screening trials.
- Treatment trials.
- Multi-arm multi-stage (MAMS) trials.
- Cohort studies.
- Case control studies.
- Cross sectional studies.
Legislation for Clinical Trials in India
- In India, clinical trials are governed by the New Drugs and Clinical Trials Rules, 2019 (NDCT Rules); the Drugs and Cosmetics Act, 1940; Medical Council of India Act, 1956; and Central Council for Indian Medicine Act, 1970.
- Central Council functions under the Ministry of Health and Family Welfare.
- Drugs Controller General of India oversees and approves these trials and operates under the Central Drugs Standard Control Organisation (CDSCO), which is accountable to the Ministry of Health and Family Welfare.
- The Indian Council of Medical Research (ICMR) governs the ethical conduct of doctors and scientists, while the Pharmacovigilance Program of India tracks drug-related harm reports.
- Each state has its own regional regulatory agencies with some involvement in trial governance.
- In response to the 2013 case Swasthya Adhikar Manch v. Union of India in the Supreme Court of India, various government agencies reformed regulations to enhance the ethics of clinical trials.
Drugs and Cosmetics Rules, 1945:
- Before introduction of NDCT Rules, clinical trials used to follow Schedule Y of the Drugs and Cosmetics Rules, 1945 (D&C Rules).
- However, under the rules there were concerns regarding patient safety and compensation in case of adverse effects prompted the need for change.
- In 2012, The Supreme Court had addressed regulatory aspects of clinical trials and has emphasized its safety, efficacy, innovation, and medical needs.
- In view of this, amendments were made to the D&C Rules in 2013 to regulate clinical trials in India.
- Rules were introduced to provide compensation to injured trial subjects and has also specified conditions for trial conduct.
- There were still deficiencies in clinical trial regulation under the Drugs and Cosmetics Rules, 1945 which has led to draft of the NDCT Rules in 2018.
New Drugs and Clinical Trials Rules, 2019
- As per the NDCT Rules 2019 and Indian Council of Medical Research guidelines, researchers must publicly document all clinical trials in the Clinical Trials Registry- India, a free and online public record system.
- The NDCT Rules brought significant changes such as it covered biomedical research, academic clinical trials, orphan drug registration, revised definitions, post-marketing studies, ethics committees, and rules for imports and manufacture of unapproved drugs.
- The NDCT Rules aimed to revitalize India’s clinical industry and enhance ethical standards in clinical trials within the country.
What are the phases of clinical trials?
- Clinical trials are conducted in four distinct stages.
- For drugs originating in India, it is obligatory to pass through all four stages of test within the country.
- Phase 0 studies usually only involve a small number of people and they only have a very small dose of a drug.
Phase-I: Clinical Pharmacology Trials
- This initial phase is also known as the “first in man” study.
- In this phase a small group of volunteers are carefully administered the new drug under close medical supervision.
- The aim of the phase is to understand how the new compound reacts within the patient’s body.
Phase-II: Exploratory Trials
- Under the second phase, the new drug is given to a group of 10-12 volunteers to assess its effects and potential side effects.
Phase-III: Confirmatory Trials
- This phase involves administering the drug to a large number of individuals, ranging from 1000 to 3000 volunteers.
- This is the most critical phase of the process.
- The data obtained from the trial is then compared to that of the standard drug to gather sufficient evidence on the new drug’s safety and effectiveness.
- If the results are positive, the data is presented to the licensing authorities to obtain a commercial license for marketing the drug to patients for specific and approved indications.
Phase-IV: Post-Marketing Phase
- After the medicine are made available to doctors for prescription, the surveillance phase commences.
- Many patients are closely monitored to identify any unexpected side effects that may arise.
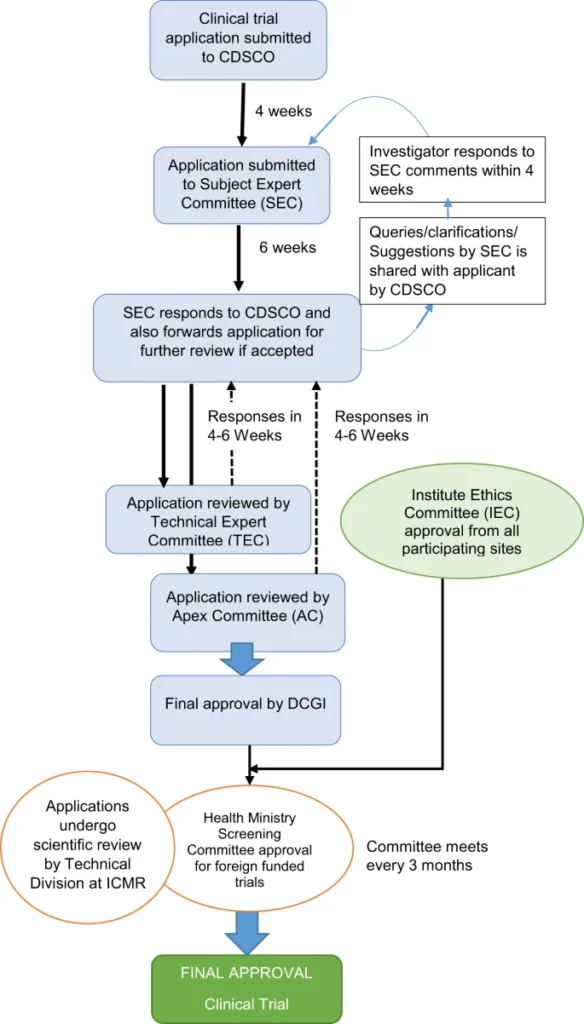
Process of Clinical Trials in India
Consent-Ethics Committees
- In order to safeguard the safety and dignity of trial subjects, the law mandates regular safety monitoring and review of clinical trials.
- To ensure a well-structured trial, a research protocol should be established.
- The World Medical Organization’s Helsinki Declaration has recommended an impartial and independent ethics committee to reviewresearch protocols.
- The Drugs and Cosmetics Rules 1945 in India has prescribed the formation of ethics committees.
- According to Schedule Y of these rules, the committee shall consist of at least sevenmembers, with the chairman being independent of the research institution.
- Other members may include social scientists, NGOs, and the community.
- However, the effectiveness of other members depends on having trained representatives who possess knowledge of medical terminology, drug side effects, and trial components.
Key Issues with Clinical Trials
- Ethical Concerns: There have been numerous instances where participants were not adequately informed about the trials they were participating in, raising ethical concerns about consent.
- Regulatory Challenges: The regulatory framework for clinical trials in India has been criticized is inadequate and poorly enforced, leading to gaps in oversight and monitoring.
- Quality and Standards: There are issues with the integrity of data collected during clinical trials have been reported, including concerns about data fabrication and manipulation.
- Compensation and Safety: Ensuring the safety of participants and providing appropriate compensation in case of adverse effects remains a significant challenge, with lack of independence for ethics committee working.
- Lack of Infrastructure: Many clinical trial sites in India lack the necessary infrastructure and resources to conduct high-quality research, affecting the overall reliability of trial results.
- Transparency and Reporting: There is often a lack of transparency in reporting the results of clinical trials, with many studies not being published or their outcomes not being disclosed to the public.
- Bias in clinical trials: It is a human tendency to form impressions that are not based on actual data, which can lead to serious cognitive errors, such as selection bias, performance bias, etc.
- Compliance with International Standards: India is a signatory to the Declaration of Helsinki but that is voluntary in nature and lack regulatory mechanisms.
- Declaration of Helsinki is a statement of ethical principles for medical research involving human subjects, including research on identifiable human material and data.
Way Forward
- Informed Consent and Patient Rights: Strengthen the protocols for informed consent and ensuring the protection of patient rights throughout the trial process. Clinical trial protocol shall be provided to the patient.
- Supporting Indigenous Research: Provide incentives for conducting indigenous research and developing local clinical trial protocols to address region-specific health issues.
- Adopting Advanced Technologies: Utilize advanced data management systems and artificial intelligence to improve data accuracy, analysis, and interpretation. Artificial intelligence (AI) can be used in clinical trials to improve efficiency and reduce costs.
- Trained investigator: A clinical trial should be planned and conducted by a trained investigator following the latest rules and regulations with meticulous record keeping and reporting.
- Ease of reporting: electronic Clinical Outcome Assessments (eCOA) is a technology uses a range of software and hardware components to capture and manage patient reported outcomes.
- Compliance with the “International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline”: It is an internationally agreed standard that ensures ethical and scientific quality in designing, recording and reporting trials involving human subjects.
- Promote open label clinical trial: It is a type of clinical trial in which information is not withheld from trial participants and thus must be promoted for better transparency.
- Medical device clinical trials (MDCTs): They are investigations or examinations undertaken to assess the safety or the performance of a medical device in terms of its use in treatment, prevention or diagnosis of diseases in human subjects.
Conclusion
Clinical trial design is an important aspect of interventional trials that serves to optimize, ergonomize and economize the clinical trial conduct. The placebo effects are often used in clinical trials to improve the efficiency of their drug or vaccines, which shall be curbed or controlled. A clinical trial is more valuable when sensitivity is higher than specificity. India offers infrastructure, easy policies (in 2019) and the government has propensity to facilitate more companies considering Indian market as a ground for clinical trial, such as those given for Covaxin. However, adverse events in clinical trials must be managed by India via effective policies.
Ref:
FAQs(frequently asked question)
Define clinical trial.
Clinical trials refer to planned research studies conducted on human participants to explore biomedical or behavioral interventions in order to address questions related to new treatments like novel vaccines, drugs, dietary choices, dietary supplements, and medical devices.
Name a clinical trial in which blood is transfused from recovered COVID-19 patients to a coronavirus patient who is in critical condition?
Convalescent plasma is a transfusion of blood plasma from someone who has recovered from COVID-19.
What is blinding in clinical trials?
Blinding is a procedure in which one or more parties in a trial are kept unaware of which treatment arms participants have been assigned to, i.e. which treatment was received. Its types include- open label, single blind, double blind, triple blind.
What are the 4 phases of clinical trials?
4 phases of clinical trials are- Clinical Pharmacology Trials, Exploratory Trials, Confirmatory Trials and Post-Marketing Phase.
What are pre-clinical trials?
It is a stage of research that begins before clinical trials (testing in humans) and during which important feasibility, iterative testing and drug safety data are collected, typically in laboratory animals.
What is decentralized clinical trial (DCT)?
In a decentralized clinical trial (DCT), some or all of a clinical trial’s activities occur at locations other than a traditional clinical trial site.
What is double blind clinical trial?
It is a type of clinical trial in which neither the participants nor the researcher knows which treatment or intervention participants are receiving until the clinical trial is over.
What is a phase 2 clinical trial?
It is “Exploratory Trials” under which new drug is given to a group of 10-12 volunteers to assess its effects and potential side effects.
What is randomization in clinical trials?
Randomization is the process of assigning participants to treatment and control groups, assuming that each participant has an equal chance of being assigned to any group.
What is Clinical Trial Agreement (CTA)?
Clinical Trial Agreement (CTA) is a legally binding agreement that manages the relationship between the sponsor that may be providing the study drug or device, financial support, proprietary information and the institution that may be providing results, publication and input.
What is interventional trial?
In interventional trials, participants are assigned to receive one or more interventions, so that researchers can evaluate the effects of the interventions on biomedical or health-related outcomes.