Osmosis, in simple terms, is the movement of water from an area of high concentration to one of low concentration through a semipermeable membrane that blocks dissolved substances. It’s a natural balancing process that influences the movement of water in all living organisms, from plant roots sucking up nutrients to our skin wrinkling in water. In this article, you will learn about meaning and definition of Osmosis, different types of Osmotic Solutions, what is Osmotic pressure, its Mechanism, Types, Significance, Examples etc. It is very important topic for GS Paper-3 Science & Technology of UPSC CSE Exam. To explore more interesting UPSC Science & technology concepts of Class 9 and Class 12 similar to Osmosis, check out other articles of and IAS Notes of IASToppers.
Table of Content
- What is Osmosis?
- Osmotic Solutions
- Osmotic pressure
- Mechanism of osmosis
- Types of Osmosis
- Significance of Osmosis
- Examples of Osmosis
- FAQs on Osmosis
What is Osmosis?
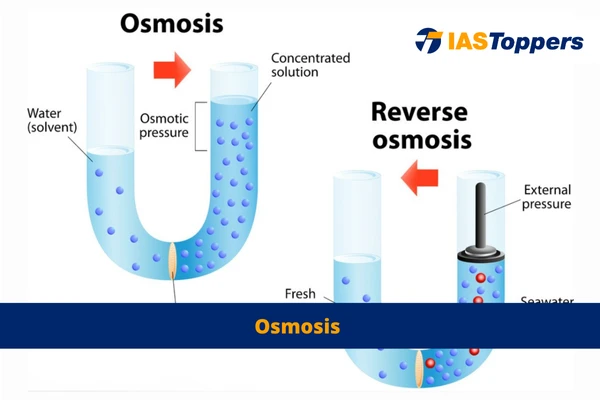
- Osmosis is a unique diffusion process where water molecules travel from an area with high water concentration to an area with low water concentration via a semipermeable membrane.
- Simply put, osmosis is the natural flow or dispersion of water or other solvents across a semipermeable membrane.
- Semipermeable membrane are membranes that blocks the passage of dissolved substances—i.e., solutes.
- The net direction and rate of osmosis depends on both the pressure gradient and concentration gradient.
- Water migrates from an area of high chemical potential (or concentration) to an area of low chemical potential until a balance is achieved.
- At this point of equilibrium, both compartments should have equal water potential.
- The general term osmose (now osmosis) was introduced in 1854 by a British chemist, Thomas Graham.
- Osmosis process was first thoroughly studied in 1877 by a German plant physiologist, Wilhelm Pfeffer.
Osmotic Solutions
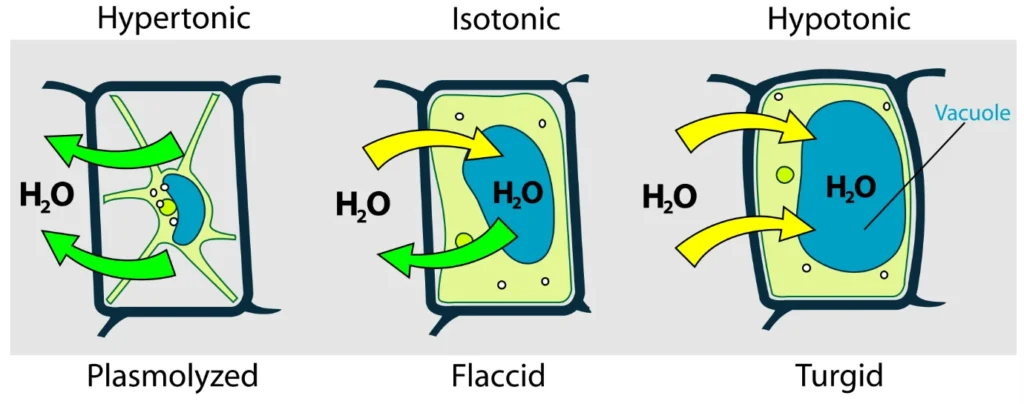
- Isotonic: In this type of solution, the water and solute concentrations are identical to those within a cell. The cell remains stable in an isotonic solution because there is no water movement into or out of the cell.
- Hypotonic: In this type, the outside solution has less solute concentration than the cell’s interior. The cell enlarges as water penetrates the cell.
- Hypertonic: In a hypertonic solution, the solute concentration is higher outside than inside the cell. The cell’s water moves out, leading to the cell’s protoplasm shrinking and gathering in the cell’s center.
Osmotic pressure
- If a solution is separated from the pure solvent by a semi-permeable membrane (permeable to the solvent but not the solute) the solution tends to passes through the membrane into the solutionand thus diluting it.
- To halt this process, the solution’s pressure needs to be increased by a particular measure, known as the osmotic pressure.
- For dilute solutions, it has been found experimentally that osmotic pressure is proportional to the molarity, C of the solution at a given temperature T.
Thus: Π = C R T
Here,
Π is the osmotic pressure
R is the gas constant
T is the temperature
Mechanism of osmosis
- The phenomenon of osmosis may be observed by dividing a container into two equal volumes with a semipermeable membrane.
- Above fig. shows a scenario where the left compartment has pure water, while the right one has a weak glucose solution.
- Water moves from the left to the right compartment, causing the level on the right to rise.
- When the right side’s solution level increases, pressure drives the water back across the membrane.
- Ultimately, equilibrium is established in the system, where the rates of water movement in both directions through the membrane become equivalent, and the water level on the right-side ceases to rise.
- The amount of pressure needed to reach this state of balance (i.e., to stop the process of osmosis) is termed as the osmotic pressure, which is an inherent attribute of the solution.
Types of Osmosis
- Endosmosis: This occurs when a cell is in a hypotonic solution and water molecules migrate into the cell.
- Exosmosis: This is the reverse scenario, where water molecules exit a cell when it is situated in a hypertonic solution, causing the cell to become plasmolyzed or flaccid.
Significance of Osmosis
- Osmosis is important in transporting nutrients and release of metabolic waste products.
- It helps in the drawing up of water from the ground and transport it to the upper portions of the plant via the xylem.
- Osmosis ensures a stable internal environment in organisms by balancing water and intercellular fluid levels.
- It maintains the turgidity of cells and hence the shape or form of the organs is maintained.
- Despite the regular water loss from transpiration, plants manage to maintain their hydration level, due to osmosis.
- Osmosis governs the spread or diffusion of water from cell to cell.
- The regulation of plant and plant part movements, including the opening and closing of stomata, is controlled by cell turgor that is induced by osmosis.
- Dehiciesnce of fruit and sporangia occurred with the help of osmosis.
- The resistance of plants to drought and frost increases with increase in osmotic pressure.
- The germination of seeds is significantly influenced by osmosis.
- Osmosis facilitates the cell’s uptake of various ions.
Examples of Osmosis
- Osmosis assists in the intake of nutrients and water from the soil. With a higher concentration in plant roots than the surrounding soil, water naturally flows into the roots.
- The opening of stomata is done by osmosis. As water concentration rises, guard cells absorb more water, swell, and cause the stomata to open.
- The impact of cholera on humans is an example of osmosis’ role. As the number of bacteria increases, they reverse the water flow, decreasing water absorption and leading to diarrhea (that is caused by cholera).
- The wrinkling of fingers after prolonged immersion in water is due to water flowing into cells via osmosis.
- When a fish from freshwater or saltwater habitats is put in water with different salt concentrations, it dies due to an imbalance of water entry or exit in its cells, highlighting the importance of osmosis.
Conclusion
Osmosis is a fundamental biological and chemical process where water molecules move across a semipermeable membrane from a region of lower solute concentration to one of higher solute concentration. This movement continues until the solute concentrations on both sides of the membrane are equal, or the system reaches an equilibrium. Osmosis is crucial for maintaining the balance of fluids in various environments, from biological cells to industrial processes.
Osmosis plays an essential role in the survival of living organisms. It helps regulate fluid balance in cells, aids in the transport of nutrients and waste products, and is involved in many physiological processes, including the stabilization of blood pressure and the proper functioning of nerve and muscle cells.
Understanding osmosis and its implications allows scientists and medical professionals to better manage and treat conditions related to fluid balance and cellular function, highlighting its significance in science and medicine.
Ref:Source-1
| Other Articles in Science & Tech | |
| Dengue | Aerosols |
| Indian Mathematicians and their Contributions | Liquid Nano Urea |
| Quantum Computers | Carbon Dating |
FAQs (Frequently Asked Questions)
What are advantages of osmosis?
Osmosis offers several advantages including maintaining hydration levels in plants despite water loss, aiding in nutrient transport, balancing water and intercellular fluid levels in organisms, and contributing to the germination of seeds.
What is Osmotic gradient?
An osmotic gradient is the difference in concentration across a semipermeable membrane, highlighting the variance in the concentration of a specific dissolved particle in a solution.
What is the difference between Osmosis and Diffusion?
While both osmosis and diffusion involve the movement of molecules from high to low concentration, osmosis specifically refers to the movement of water across a semipermeable membrane, whereas diffusion can involve any type of molecules and doesn’t require a membrane.
Is osmosis a passive or active process?
Osmosis is a passive process as it naturally occurs without the need for energy, moving water from an area of high concentration to one of low concentration.
What are the factors affecting osmosis?
Factors affecting osmosis include the concentration of solutes in the solution, temperature, and pressure, all of which can influence the rate and direction of water movement.
What is the difference between osmosis and reverse osmosis?
While osmosis refers to water moving from a high to low concentration, reverse osmosis is the opposite – it uses pressure to move water from a low to high concentration, typically used in water purification processes.
What is the difference between difference between osmosis and imbibition?
While osmosis involves the movement of water across a semipermeable membrane, imbibition is the absorption of water by solid particles leading to their swelling, with no membrane involved.