Ozone layer depletion is a consequence of the interaction between chlorine and bromine atoms with ozone in the stratosphere. Certain substances known as ozone-depleting substances (ODS), including chlorofluorocarbons (CFCs) and halons, release chlorine and bromine upon exposure to intense UV light in the stratosphere, contributing to the ongoing ozone depletion phenomenon. A single chlorine atom has the potential to eliminate more than 100,000 ozone molecules before its departure from the stratosphere.
Ozone Layer Depletion will be helpful for UPSC IAS Exam preparation. GS Paper-3 Environment.
Table of Content
- What is Ozone?
- What is Ozone Layer?
- What is Ozone hole?
- What is Ozone Layer Depletion?
- Causes of ozone layer depletion
- Effects Of Ozone Layer Depletion
- Measures to prevent ozone (O3) layer depletion
- Frequently Asked Questions
What is Ozone?
- Ozone, which consists of three oxygen atoms (O3), exists naturally in small quantities in both the upper atmosphere and near the Earth’s surface.
- Ozone protects life on Earth from the Sun’s ultraviolet (UV) radiation.
- Ozone has a distinctive and sharp odor reminiscent of chlorine, and can sometimes be smelled after a thunderstorm, when lightning zaps oxygen molecules apart.
- This property is what gives ozone its name, after the Greek word ozein, meaning “to smell”.
- The total mass of ozone in the atmosphere is about 3 billion metric tons, which is only 0.00006 per cent of the atmosphere.
- The ozone found in the stratosphere is a result of the natural process involving the interaction of solar ultraviolet (UV) radiation and molecular oxygen (O2).
- The ozone found in the lower atmosphere or at ground level is created by volatile organic compounds (VOC) and nitrogen oxides (NOx).
- Ozone affects life on Earth in either good or bad ways, depending on where it is in the atmosphere.
What is Ozone Layer?
- Definition: The ozone layer refers to a layer that is present in the Earth’s stratosphere which contains relatively high concentrations of ozone.
- It occurs naturally in small amounts in the upper atmosphere and protects life on Earth from the Sun’s ultraviolet (UV) radiation.
- About 90% of the planet’s ozone is in the ozone layer, which shields life on Earth from harmful solar ultraviolet (UV) radiation.
- The layer is important because excessive exposure to UV radiation is harmful to agriculture, the marine food chain, and causes skin cancer and eye problems.
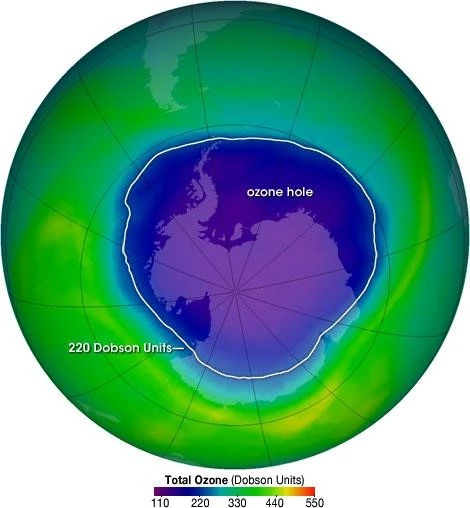
What is Ozone hole?
[AT: Diagram of Ozone Hole]
- The ozone hole is a loss of stratospheric ozone over Antarctica, defined as the size of the region with total ozone below 220 Dobson units (DU).
- Dobson Units are a unit of measurement that refers to the thickness of the ozone layer in a vertical column from the surface to the top.
What is Ozone Layer Depletion?
- Ozone layer depletion: The gradual thinning of the Earth’s ozone layer in the upper atmosphere caused by the release of certain chemical compounds containing chlorine or bromine from human activities and industrial processes.
- Ozone-depleting compounds (ODS): Substances that contribute to the depletion of the ozone layer, including hydrochlorofluorocarbons (HCFCs), chlorofluorocarbons (CFCs), and other halocarbons.
- When chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules.
- One chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere.
Causes of Ozone Layer Depletion
The ozone (O3) layer can undergo destruction due to both natural and man-made causes.
Natural causes of ozone depletion
- Natural causes of ozone depletion include hydrogen oxide (HOx), methane (CH4), hydrogen gas (H2), and nitrogen oxides (NOx).
- During volcanic eruptions, significant amounts of chlorine may be released into the stratosphere through chlorine monoxide (ClO) reactions.
- Stratospheric aerosols, which are tiny particulate matter, can also contribute to ozone destruction.
Human activity-related causes of ozone depletion
- Human activities are another cause of ozone depletion, particularly events that release chlorine atoms into the atmosphere.
- Chlorofluorocarbons (CFCs), commonly used as refrigerants and in spray cans, are highly damaging agents that release chlorine atoms in the stratosphere.
- In the stratosphere, chlorine atoms from CFCs react with ozone to form chlorine monoxide and oxygen molecules.
Ozone-depleting chemicals and their uses:

Effects Of Ozone Layer Depletion
Harmful effects on human beings:
- Increased susceptibility to skin cancer
- Increased risk of cataracts
- DNA damage
- Corneal damage
- Increased likelihood of retinal diseases
- Weakened immune systems
Harmful effects on plants:
- Inhibition of photosynthesis
- Impaired metabolism
- Repressed growth
- Cell Destruction
- Increased risk of mutations
- Decline in forest productivity
Harmful effects on other organisms:
- High sensitivity of marine and freshwater organisms to UV rays
- Vulnerability of fish larvae to UV exposure
- Severe damage to plankton populations
- Adverse effects on fish, shrimp, and crab larvae
Harmful effects on non-living materials:
- Accelerated breakdown of paints
- Increased degradation of plastics
- Disruption of temperature gradients in the atmosphere
- Altered atmospheric circulation patterns and climatic changes
Measures to prevent ozone (O3) layer depletion
- Global efforts and initiatives by the international community, such as the Helsinki (1989) and Montreal (1990s) conventions and protocols, have played a significant role in raising awareness and taking action on ozone layer depletion.
- A crucial step in combating this issue is the complete prohibition of the use of ozone-depleting chemicals, including CFCs.
- As a temporary measure, the use of HCFCs (Hydrochloric fluorocarbons) is being suggested as an alternative to CFCs, as they have a relatively lower impact on the ozone layer.
- However, it’s important to note that HCFCs are not entirely ozone-safe.
Conclusion
Ozone layer depletion results in harmful effects on humans, plants, organisms, and non-living materials. International efforts like the Helsinki and Montreal conventions have played a significant role, emphasizing the complete prohibition of ozone-depleting chemicals and exploring alternatives such as HCFCs with lower impact.
Ref:Source-1
| Other Articles in Environment & Disaster Management | |
| Kyoto protocol | Smog |
| Carbon FootPrint | Central Pollution Control Board (CPCB) |
| Black Carbon | World Meteorological Organization |
FAQs (frequently asked question)
Where is ozone layer found?
The Ozone layer is found in the Stratosphere, between approximately 15-40 kilometers (10-25 miles) above the Earth’s surface.
How is ozone formed in the atmosphere?
The atmosphere witnesses a series of chemical reactions, facilitated by sunlight, leading to the formation of ozone. In the stratosphere, ultraviolet radiation from the Sun splits oxygen molecules (O2), initiating the process. Within the lower atmosphere (troposphere), a distinct set of reactions, involving both natural gases and pollutants, contribute to the creation of ozone.
Is ozone a greenhouse gas?
Ozone (O3) is technically a greenhouse gas, but it is helpful or harmful depending on where it is found in the atmosphere.
What is ozone layer depletion?
Ozone layer depletion is the gradual thinning of the Earth’s ozone layer in the upper atmosphere caused by the release of chemical compounds containing gaseous chlorine or bromine from industry and other human activities.
What are the effects of ozone layer depletion on human health?
When the ozone layer is depleted, humans are exposed to skin diseases, cataracts, cancer, and a weakened immune system.
Which day is marked as International Ozone Day?
In 1994, the United Nations General Assembly (UNGA) declared 16th September as World Ozone Day.
What are ozone depleting substances?
The ozone layer is depleted by substances including chlorofluorocarbons (CFCs), halon, carbon tetrachloride (CCl4), methyl chloroform (CH3CCl3), hydrobromofluorocarbons (HBFCs), hydrochlorofluorocarbons (HCFCs), methyl bromide (CH3Br), bromochloromethane (CH2BrCl) etc.