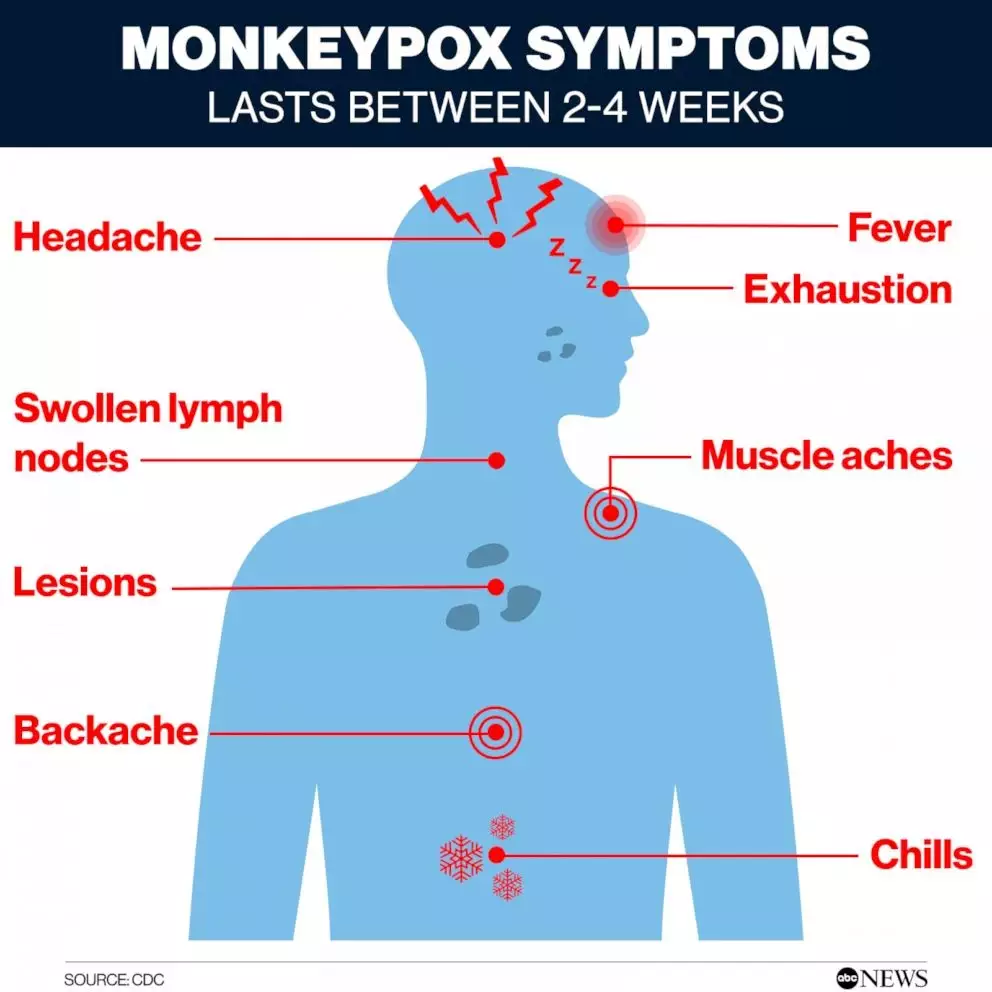
The World Health Organization (WHO) has recently prequalified the MVA-BN vaccine to address the global health emergency posed by mpox, previously known as monkeypox.
About Mpox:
- Definition: Mpox is a zoonotic disease caused by the monkeypox virus (MPXV).
- Pathogen: An enveloped double-stranded DNA virus belonging to the Orthopoxvirus genus.
- Transmission: Spread through close contact with infected individuals or animals and from mother to foetus during pregnancy.
- Symptoms: Includes skin rash or mucosal lesions, fever, headache, and swollen lymph nodes.
- Public Health Concern: Declared a public health emergency of international concern (PHEIC) by WHO in 2022 and 2024.
WHO’s MVA-BN Vaccine Prequalification:
- Vaccine Name: MVA-BN, developed by Bavarian Nordic A/S.
- Significance:
- First vaccine against mpox to be prequalified by WHO.
- Aims to facilitate access for communities with urgent needs to reduce transmission and control the outbreak.
- Approval Process: Based on manufacturer data and evaluation by the European Medicines Agency.
Effectiveness:
- Single-dose: Approximately 76% effective if given before exposure.
- Two-dose schedule: Approximately 82% effective.
WHO Recommendations:
- Use in High-Risk Groups: Recommended for individuals at high risk of exposure during outbreaks.
- Single-Dose Consideration: In supply-constrained situations, single-dose administration is suggested, with ongoing data collection on safety and effectiveness.
Importance of WHO Vaccine Prequalification
- Genesis: Established in 1987 to ensure vaccine quality for UN purchasing agencies.
Prequalification Process:
- Includes vaccines that show positive outcomes based on data evaluation and inspections.
- Inclusion does not imply WHO approval of manufacturing sites, such authority lies with National Regulatory Authorities (NRAs).
Benefits:
- Accelerates vaccine procurement by governments and international organizations.
- Supports national regulatory authorities in expediting vaccine approvals.
- Expanded Immunization Program: Contributes to WHO’s efforts to ensure universal vaccine access for at-risk populations.
Ref: Source
| UPSC IAS Preparation Resources | |
| Current Affairs Analysis | Topperspedia |
| GS Shots | Simply Explained |
| Daily Flash Cards | Daily Quiz |
Frequently Asked Question:
What is mpox?
Mpox is a zoonotic disease caused by the monkeypox virus, characterized by skin rashes, fever, and swollen lymph nodes.
What is the MVA-BN vaccine?
The MVA-BN vaccine, developed by Bavarian Nordic, is the first WHO-prequalified vaccine against mpox.
How effective is the MVA-BN vaccine?
A single dose is approximately 76% effective, and a two-dose schedule boosts effectiveness to about 82%.
Who is recommended to take the MVA-BN vaccine?
The WHO recommends it for individuals at high risk of exposure to mpox during outbreaks.
What is the significance of WHO prequalification for vaccines?
WHO prequalification ensures the vaccine meets quality standards, facilitating quicker access in health emergencies.